
BIO 190 - SUPPLEMENTAL BIOLOGY WORKSHOP I
Cellular Respiration
____.) Fill in the boxes with the three metabolic stages of respiration (a-c). Indicate whether the ATP is produced by substrate-level phosphorylation or oxidative phosphorylation (d-f). Label the arrows that show the flow of electrons via NADH.

____.) a. Fill in the table below by naming the sites of the respiratory stages and filling in the appropriate inputs and outputs of each respiratory stage.

b. After completing the table, complete the following ATP tally sheet for Eukaryotic Cellular Respiration.

c. Why is there a difference in the net yield of ATP per NADH produced in glycolysis versus the net yield of ATP per NADH produced in the Krebís Cycle? d. Explain the difference in the number of ATPís produced in prokaryotes versus eukaryotes during aerobic respiration. Be specific as to which step and site in the cell that this occurs.
____.) In glycolysis:
a. acetyl CoA is formed
b. NADH is oxidized
c. glucose is split to form to molecules of pyruvate
d. acetate is joined to oxaloacetate to form citrate
e. energized electrons are passed along the electron transport chain
____.) In glycolysis, glucose is broken down into 2 pyruvate molecules. Which of these molecules has a higher free energy? Why?
____.) During the Pre-Krebís reaction, pyruvate is converted into acetyl CoA.
a. What is released during this reaction?
b. Does pyruvate or acetyl CoA have more free energy? Why?
c. In this reaction, what is being reduced? What is the reducing agent?
____.) What product from the pre-Krebís reaction crosses into the mitochondria to become a reactant in the Krebís cycle?
____.) Glucose is catabolized to pyruvate, which is then converted to acetyl CoA before entering the Krebís cycle. Which of the following statements is true?
a. Acetyl CoA is in more reduced form than glucose
b. Pyruvate is more energy rich than glucose
c. Pyruvate accepts electrons from lysed glucose
d. 2 molecules of acetyl CoA have less energy than in one molecule of glucose
____.)
a. List the following in order of highest energy content to the lowest energy content.
Pyruvate
Acetyl CoA
Oxaloacetate
Glucose
b. Since these molecules decrease in energy as they move through the respiration steps, what gains the energy and how?
____.) One acetyl CoA molecule would produce how many of the following?
a. carbon dioxides =
b. NADH =
c. NAD+ =
d. ATP =
e. FADH2 =
f. FAD =
____.) In the following drawing:
a. Label the compartments and membranes of the mitochondria.
b. Fill in the appropriate blanks where FADH2 and NADH are oxidized.
c. Draw the pumping of the H+ across the membrane.
d. Draw the flow of electrons through the ETC.
e. Draw the flow of the H+ required to synthesize ATP.

____.) At which protein do the electrons have the most energy?
____.) What is the final electron acceptor in aerobic respiration?
____.)
a. In order for the ETC to produce ATP, an ADP must be phosphorylated. Is this reaction endothermic or exothermic?
b. What is the driving force for this reaction?
c. How does the mitochondrial membrane generate and maintain an H+ gradient?
d. Chemiosmosis is said to be the coupling mechanism for oxidative phosphorylation. What processes does it couple? (HINT: Energy liberated from exothermic reactions is utilized to drive endothermic (non-spontaneous) reactions. Thus, these reactions must be coupled together.
____.) The following equations are from the aerobic respiration pathways. Circle the molecules which are being reduced and put a box around the molecule which is being oxidized. Also, label the oxidizing agent with an ĎOí and the reducing agent with an ĎRí.
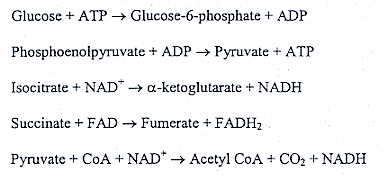
____.) For each of the following pairs, circle the molecule with the greatest free energy. What is the connection between all of the molecules which you have circled?

____.)
a. During which stage(s) and where is NADH oxidized in aerobic respiration?
b. During which stage(s) and where is FAD reduced?
____.) What is the final electron acceptor(s) in prokaryotic anaerobic respiration?
____.) What is the net ATP yield for one glucose molecule undergoing anaerobic respiration?
____.) List two basic types of fermentation. What are the end products for each of these processes?
____.)
a. What is the final electron acceptor in lactic acid fermentation?
b. What is the final electron acceptor in alcohol fermentation?
____.)
a. What is the net ATP yield for one glucose molecule undergoing fermentation?
b. Is the ATP formed in fermentation by substrate phosphorylation or by oxidative phosphorylation?
____.) What is the determining factor that makes pyruvate undergo fermentation or proceed into aerobic metabolism in facultative anaerobes or muscle cells in eukaryotes?
____.) Discuss the similarities and differences between aerobic respiration and fermentation.
____.) Although NADH and FADH2 are energy rich molecules, why must they be converted to ATP?
____.) Suppose the electron carrier NAD+ reduced to NADH in glycolysis did not later give up electrons in fermentation. What would happen to the fermentation process?
____.) A century ago, Louis Pasteur, the great French biochemist, investigated the metabolism of yeast, a facultative anaerobe. He observed that the yeast consumed sugar at a much faster rate under anaerobic conditions than it did under aerobic conditions. Explain this Pasteur effect, as the observation is called (Campbell, 1996).
____.) In the 1940ís, some physicians prescribed low doses of a drug called dinitrophenol (DNP) to help patients lost weight. This unsafe method was abandoned after a few patients died. DNP uncouples the chemiosmotic machinery by making the lipid bilayer of the inner mitochondrial membrane leaky to H+. Explain how this causes weight loss (Campbell, 1996).
____.) Sitting here reading this question, your calf muscle consumes 216 ATP/hour.
a. How much glucose must be catabolized/hr to keep up with the energy demands of your calf muscles?
b. How many molecules of carbon dioxide does your calf muscle produce in the two hours you are sitting in class?
c. How many water molecules does your calf muscle produce in two hours?
d. How many oxygen molecules does your calf muscle require in the two hours?
e. When the bell rings, you sprint to your car which of course is at least one mile away (parking stinks!). About halfway to your car, your calf muscles begin to get tight and hurt. Explain what has happened during your sprint to the car.
f. Your house is abut 30 minutes away from campus and by the time you get home your legs feel better and you are breathing normally. Explain what has happened during your drive home.
____.) If oxygen is reduced in the atmosphere so that it can not keep up with the energy demands of the body:
a. What happens to the production of ATP in the ETC?
b. What happens to the concentration of citrate, an intermediate in the Krebís cycle?
c. If the citrate concentration increases, what happens to the activity of the enzyme phosphofructose kinase, an enzyme used to catalyze a reaction early in the glycolysis pathway?
d. If citrate was used to reduce the enzyme activity of phosphofructose kinase, what type of inhibitor do you think it would be? Explain.
e. What type of inhibition is this (refer to part d)?
f. What product of aerobic respiration is also responsible for the inhibition of this enzyme?
____.) What is the net ATP yield of one acetyl CoA going into the Krebís cycle and through the ETC?
____.) In aerobic metabolism NAD+ are constantly being reduced to NADH in glycolysis and the Krebís cycle. What is the reason we do not run out of NAD+ and FAD?
____.) Why do scientists think glycolysis is the most ancient of metabolic processes? List at least 3 reasons.
____.) Why must a comatosed quadriplegic, who cannot possibly move, need to be fed intravenously? What is the energy needed for?
© 2002-2003 Kevin Hong